A Breakthrough Is
Needed for Treating T1D
The landmark Diabetes Control and Complication Trial established prospectively a connection between HbA1c and long-term complications. [1] As a result, the goal of T1D therapy is to achieve HbA1c levels as close to the normal, healthy range of 4.0% to 5.6% as possible.
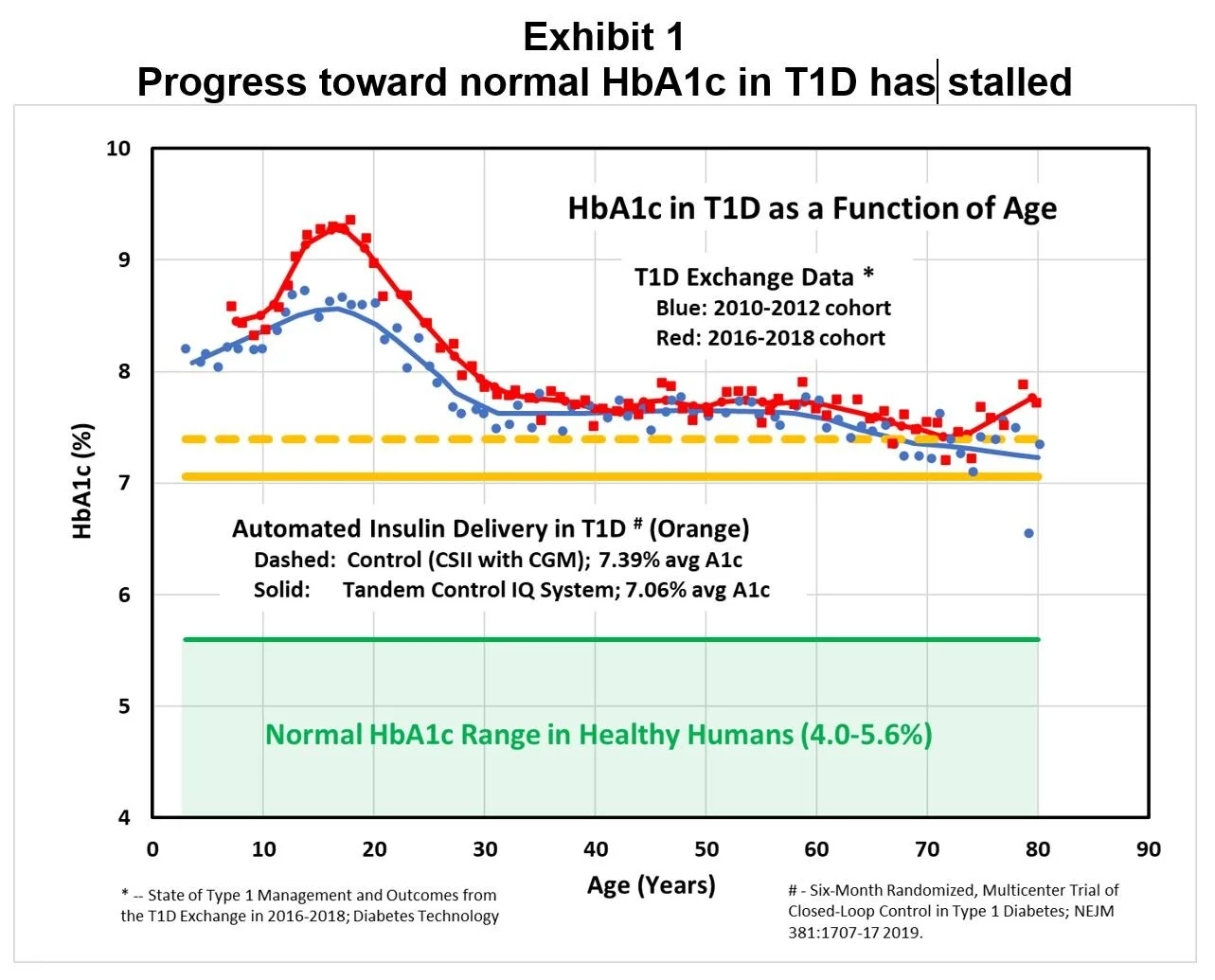
Unfortunately, data published in early 2019 from the T1D Exchange Registry indicates that progress toward this goal is stalled in the broad population of T1D patients. The T1D Exchange Clinic Network includes 81 U.S.-based endocrinology practices in 35 states, and their February 2019 report is based on data from 22,697 participants. [2] As shown in Exhibit 1, during the six years ending 2018 average HbA1c results for T1D patients did not improve (and may have deteriorated in teenagers), and average HbA1c levels were above the ADA guideline of 7%.
This disappointing situation has occurred despite continuing investments in new technologies for treating T1D, as reflected by the patients in the T1D Exchange Clinic Network. More patients are using continuous subcutaneous insulin infusion (CSII), with participation rising from 57% to 63%. And patients reported an even more striking increase in the use of continuous glucose monitoring (CGM), from 7% to 30%. Since patients participating in the Registry can be expected to be more motivated and informed than nonparticipants, the HbA1c averages in the total 1.5 million American T1D population are probably higher. [3]
This failure to achieve euglycemia in T1D has serious consequences: a 2015 study estimated that the loss of life expectancy from age 20 for a T1D patient is 11-13 years. [4] Clearly the number one goal in treating T1D is to permit patients to achieve HbA1c levels in the normal, healthy range.
To this end, Automated Insulin Delivery (AID or the “artificial pancreas”) is a primary focus of research and development aimed at improving therapy of T1D. (For 2018 review of progress toward AID, see reference [5].) AID has been shown to reduce the burden of therapy for T1D patients by automating portions of the therapy regimen; for example, a recent study in children demonstrated the AID system was associated with less time thinking about diabetes, decreased worry about blood sugars, and decreased burden in managing diabetes. [6] The system improved Time-in-Range (TIR: percentage of a 24-hour period during which CGM readings are in the range 70-180 mg/dl) from 53% without AID to 71% with AID, and mean glycemic control improved without increasing hypoglycemia.
While TIR has been correlated with the risks of retinopathy and microalbuminuria, it remains that “A1c is the only prospectively evaluated tool for assessing the risk for diabetes complications.” [7] So, how well do the AID systems perform with respect to normalizing average blood glucose levels?
To establish context for this question, Exhibit 2 compares the official TIR goal to the normal glucose excursions in healthy subjects. [8] Also shown in Exhibit 2 is the estimated Average Glucose (eAG) range needed to achieve the normal range for HbA1c of 4.0-5.6%. [9]
Exhibit 2 demonstrates that the official consensus for TIR has been set higher and more broadly than the typical healthy glucose excursion range. This was done presumably to reflect the need for a hyperglycemic “buffer,” given the risk of iatrogenic hypoglycemia. This official TIR is viewed as challenging but doable with present technology, but it does not reflect a healthy, normal range of glucose excursions.
Returning to Exhibit 1, the orange lines demonstrate the improvement in HbA1c achieved with the most advanced AID system to achieve FDA clearance in 2019, the Control-IQ by Tandem Diabetes: “This closed-loop system uses an algorithm with a dedicated hypoglycemia safety module, automated correction boluses, and overnight intensification of basal insulin delivery designed to consistently target near-normal glycemia each morning, which was compared to control subjects using their insulin pumps augmented by continuous glucose monitoring (CGM).” [10]
In this example the AID system lowered HbA1c from 7.39% to 7.06%, a reduction of 0.33%. This is about the same improvement in HbA1c shown with pramlintide treatment, which was not enough to make SYMLIN a marketing success. [11]
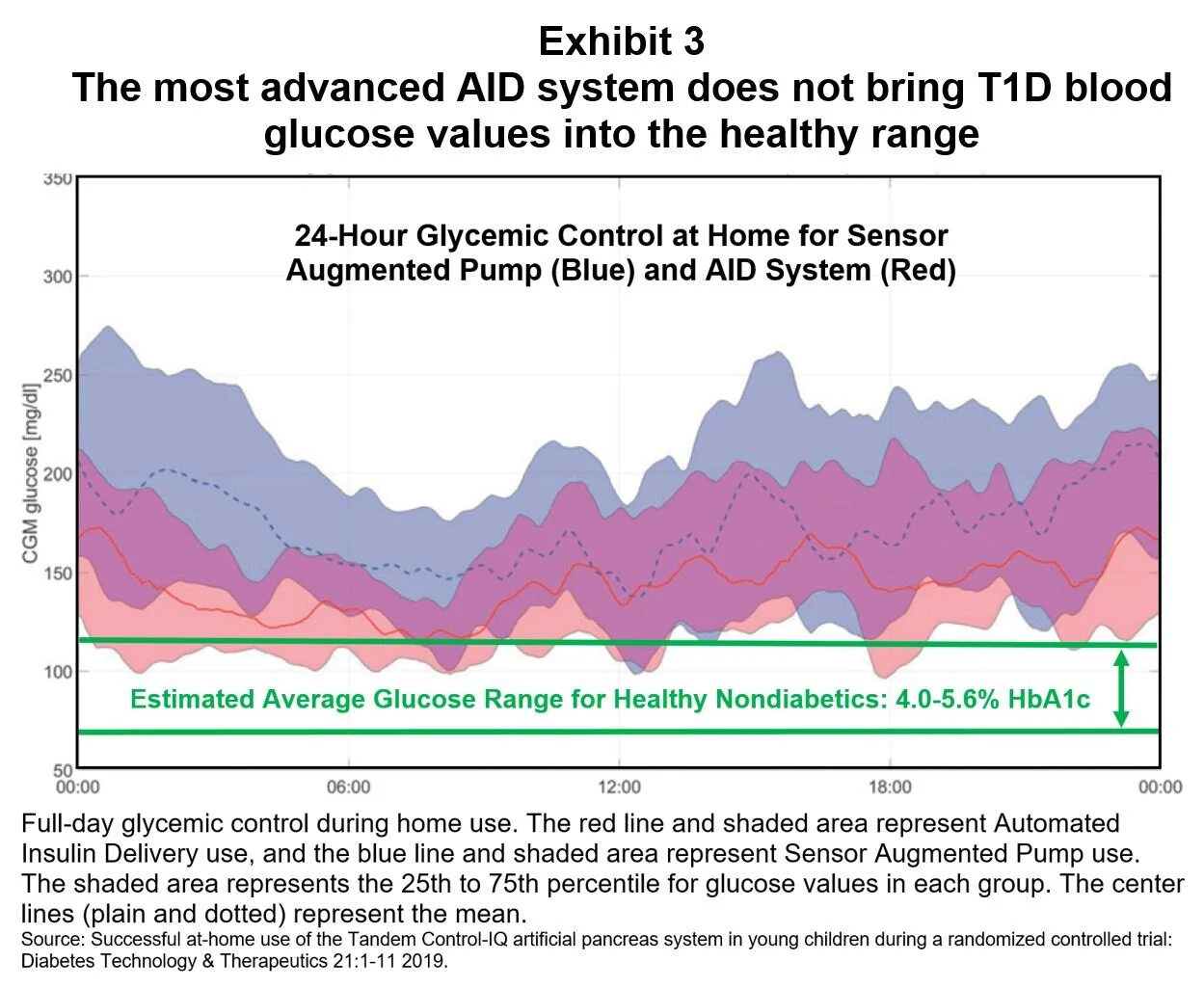
For patients in a children’s AID study, daily ambulatory glucose values were reduced, especially in the morning; however, most of the reported glucose values were above the healthy range of euglycemia (Exhibit 3). [6]
These disappointing results are widely attributed to two problems with the most convenient and least invasive route of insulin delivery, subcutaneous (SC) infusions:
Slow delivery into circulation: Diffusion through the subcutaneous tissues introduces delays to insulin action and clearance that make tight control difficult. [12] The hurdles to tight glycemic control caused by these delays are so significant that many AID studies have added meal announcements with full or partial boluses triggered by the patient to improve postprandial glucose control, thereby trading-off autonomy for performance. “(A) limitation remains on the effectiveness of AP caused by the speed of insulin action, with automated basal rate changes taking time – sometimes several hours – to show full clinical effect.” [13] Unfortunately, new insulin formulations have offered only modest improvements: A new, more rapid onset insulin resulted in an HbA1c improvement of only 0.15% in one 6-month study. [14]
Low hepatic concentrations: Because about 50% of beta-cell-secreted insulin is taken up by the liver, SC infusions cannot approach normal hepatic concentrations without causing severe hyperinsulinemia in peripheral circulation. To partially compensate for inadequate hepatic insulin, dosing practice in T1D results in patients being about 65% hyperinsulinemic on average during the diurnal cycle, which in conjunction with hyperglycemia may contribute to their relative insulin resistance (see Appendix A). Perhaps as a result of inadequate hepatic insulin, T1D patients are deficient in liver glycogen, [15] which may contribute to their glucose counterregulatory failure. Thus, intraperitoneal (IP) insulin delivery has been studied as an improvement over the SC route. In one study, changing from SC to IP delivery improved HbA1c from 8.8% to 7.2% in ten patients after 24 months. [16] But this more invasive technology is unlikely to replace SC infusions in the near term.
While AID systems are now considered the future of T1D therapy, achieving true euglycemia in T1D will require new pharmaceutical strategies beyond insulin. Our hypothesis is that appropriate therapy adjunctive to insulin may be able to compensate for the limitations of SC insulin infusions. Are there any candidates?
Endnotes
[1] The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus; N Engl J Med 329:977-86 1993.
[2] State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018; Diabetes Technology & Therapeutics 21: 1-7 2019.
[3] National Diabetes Statistics Report, 2017; https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. (T1D estimated as 5% of the 30.3 million American diabetics.)
[4] Estimated Life Expectancy in a Scottish Cohort With Type 1 Diabetes 2008-2010; JAMA 313(1):37-44 2015.
[5] Automated closed-loop control of diabetes: the artificial pancreas; Bioelectronic Medicine 4:14 2018.
[6] Successful at-home use of the Tandem Control-IQ artificial pancreas system in young children during a randomized controlled trial; Diabetes Technology & Therapeutics 21:1-11 2019.
[7] Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range; Diabetes Care 42:1593-1603 2019.
[8] Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals; Journal of Diabetes Science and Technology 1(5):695-703 2007.
[9] As calculated from https://professional.diabetes.org/diapro/glucose_calc.
[10] Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes; NEJM 381:1707-17 2019.
[11] SYMLIN Package Insert: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021332s007_S016.pdf.
[12] Intraperitoneal insulin delivery provides superior glycaemic regulation to subcutaneous insulin delivery in model predictive control-based fully-automated artificial pancreas in patients with type 1 diabetes: a pilot study; Diabetes Obes Metab 19(12):1698-1705 2017.
[13] Twelve-week 24-7 ambulatory artificial pancreas with weekly adaptation of insulin delivery settings: effect on hemoglobin A1c and hypoglycemia; Diabetes Care 40:1719-26 2017.
[14] Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1); Diabetes Care 40:943-50 2017.
[15] Impaired Net Hepatic Glycogen Synthesis in Insulin-dependent Diabetic Subjects during Mixed Meal Ingestion; J Clin Invest 95:783-787 1995.
[16] Long-term clinical evaluation of the new Accu-Chek DiaPort, a port system for continuous intraperitoneal insulin infusion: 24-month results; Poster 941-P 74th scientific sessions of ADA 2014.