Intrahepatic Islet Transplants
Demonstrate Correct Amylin Replacement
The most obvious way to administer amylin replacement therapy in T1D is the use of pramlintide. A less obvious approach has been tested in Phase 3 clinical trials: intrahepatic islet transplants.
“Special enzymes are used to remove islets from the pancreas of a deceased donor. The islets are purified and counted in a lab. On average, about 400,000 islets are transplanted in each procedure. The islet transplant infusion procedure involves inserting a thin, flexible tube called a catheter through a small cut in the recipient’s upper abdomen. A radiologist uses x-rays and ultrasound to guide the catheter into the portal vein of the liver. The islets are slowly infused through the catheter and into the liver by gravity. Alternatively, a minimally invasive open procedure can be used to directly visualize a vein near the liver to insert the catheter.” [1]
Islet transplantation is indicated for patients with T1D who suffer from disabling, severe hypoglycemia events despite optimized insulin therapy. It is NOT indicated for patients as a way of lowering HbA1c or of eliminating the need for insulin injections. Because transplants are precisely directed at correcting a potentially catastrophic failure of counterregulation, it is instructive to consider whether observed effects of transplants are predicted by our amylin circuit-breaker model.
With respect to glucose counterregulation, islet transplants have been shown to be remarkably effective.
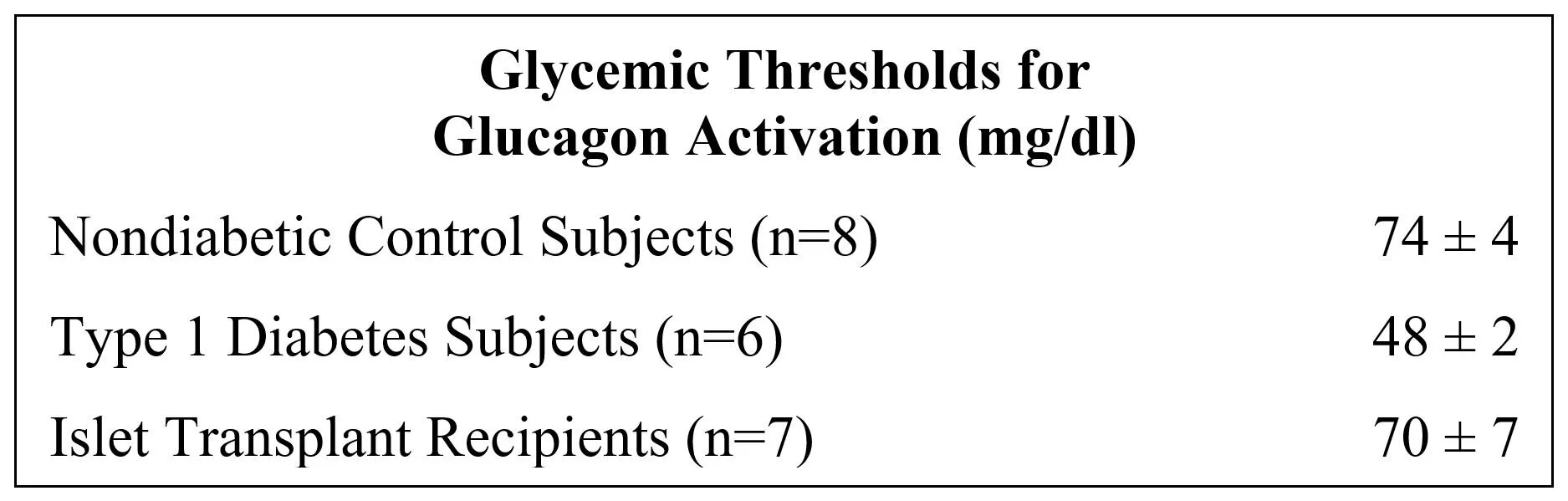
Transplants increase the glycemic threshold for glucagon activation: In a study of activation thresholds, islet transplants were found to almost restore the level observed in nondiabetic control subjects: [2]
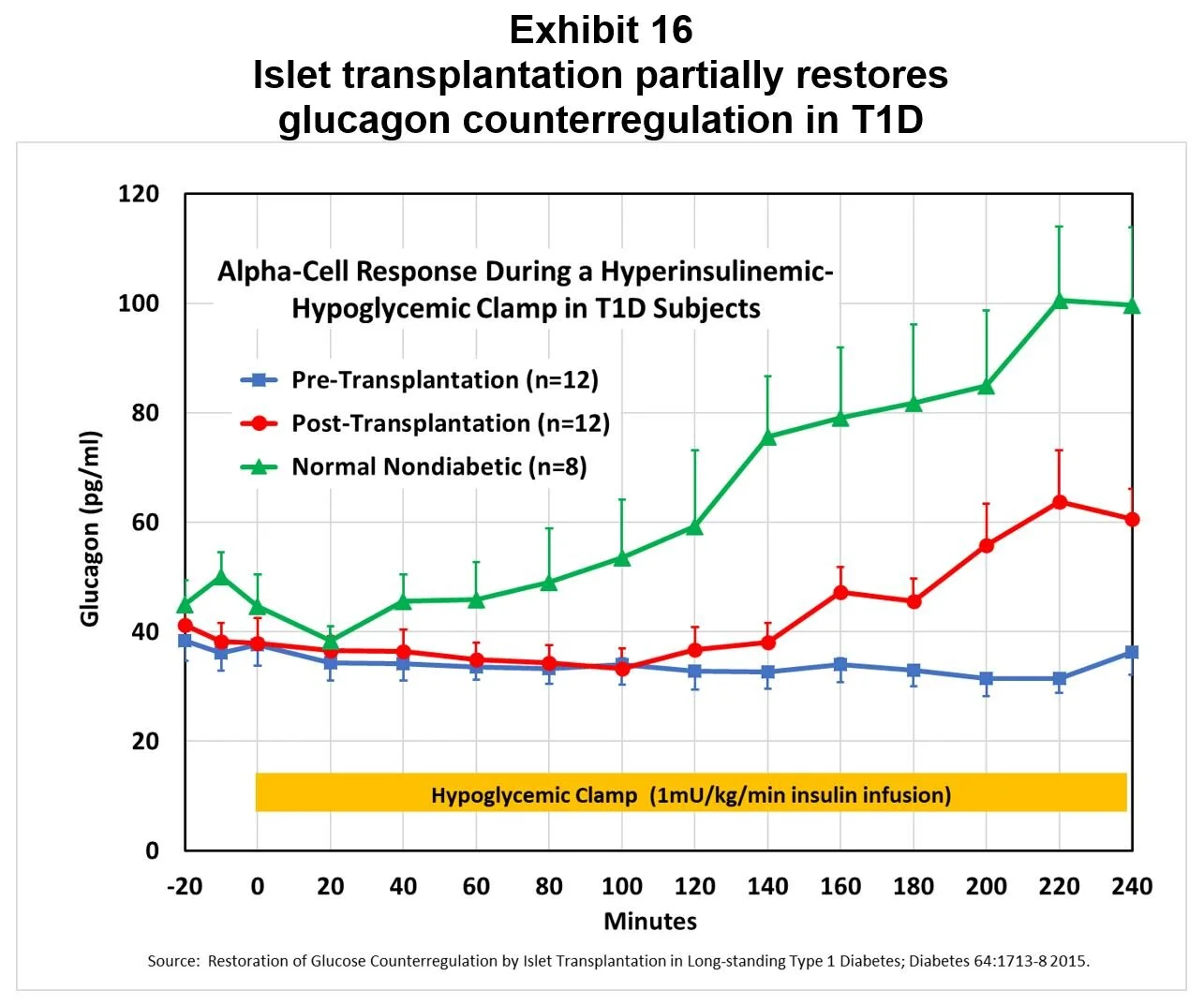
Transplants restore partial glucagon counterregulation: Another study demonstrated a partial restoration of the glucagon response to hypoglycemia, as shown in Exhibit 16. [3]
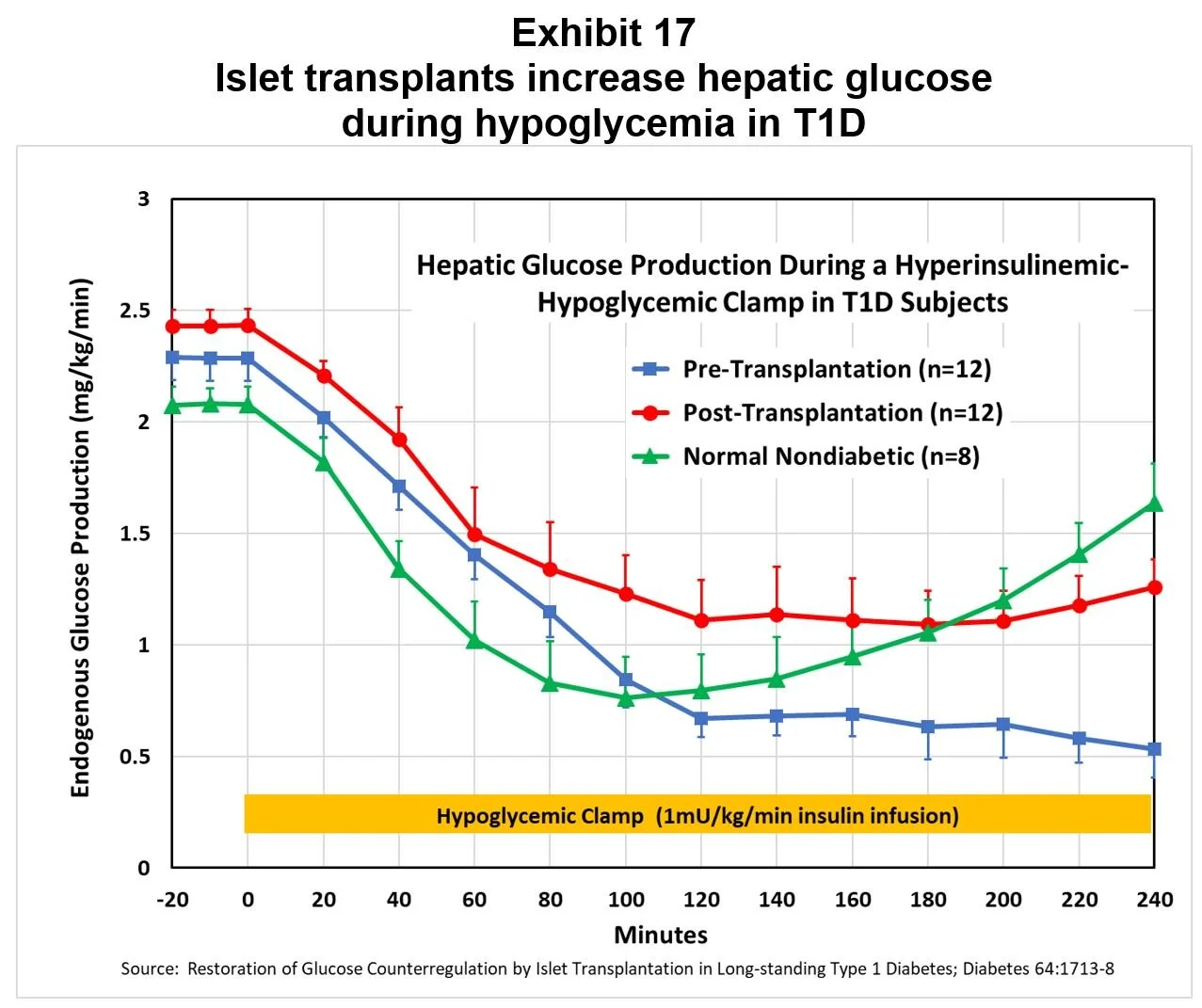
Transplants increase hepatic glucose output during hypoglycemia: The same study demonstrated islet transplants increased the supply of glucose from the liver during hypoglycemia, as shown in Exhibit 17.
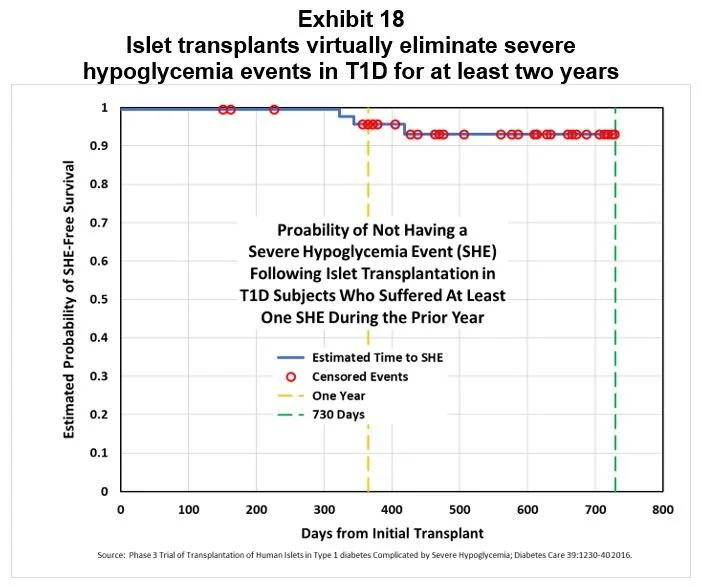
Transplants improve HbA1c and prevent serious hypoglycemia events: In a Phase 3 study of islet transplantation, recipients’ average HbA1c declined from 7.2% at baseline to 5.6% one year later. Subjects in this study had all suffered during the prior year at least one serious hypoglycemic event requiring assistance, even though they were under the care of an endocrinologist or diabetologist and had adhered to recommended glucose monitoring and insulin therapy (77% used CSII and 44% used CGM; n=48). Over the two year period following transplantation, recipients were virtually risk free of serious hypoglycemia events, as shown in Exhibit 18. [4]
Our view is that islet transplants partially restore the glucagon counterregulatory response because the transplanted beta-cells deliver a relatively normal diurnal profile of circulating amylin. At first blush, this proposal would appear to contradict our CNS-mediated circuit-breaker model, because the transplanted alpha-cells are NOT innervated and could not respond to tonic inhibition via the CNS. This apparent conflict can be easily resolved by assuming that the restored counterregulatory response occurred in the endogenous alpha-cells, not in the transplanted islets, as shown in this version of the circuit-breaker model:
We believe that, with respect to alpha-cell function, islet transplants are a physiologically correct way to deliver amylin replacement therapy, so that it restores counterregulation in patients’ own pancreatic islets. And, we think there is reason to expect appropriate delivery of pramlintide could do an even better job of restoring counterregulation:
In the studies cited above, the impaired glucagon response was attributed to the mass of surviving transplanted islets being less than normal as estimated by measurement of the beta-cell secretory capacity. If so, the tonic inhibition of alpha-cells may be muted. Correct dosing of pramlintide might resolve that deficiency.
Because the transplanted alpha-cells are denervated and therefore not subject to tonic inhibition by amylin, their unrestrained glucagon secretion may interfere with full restoration of counterregulation. Pramlintide infusions would avoid this complication.
Bottom line, we think that islet transplant restoration of counterregulation is consistent with our circuit-breaker model, and that this observation underscores the need to test our model in the clinic with pramlintide infusions.
Why is there not yet evidence from clinical studies? Is it not be reasonable to expect that, after 15-some years of pramlintide patient use, there would be some signal that amylin plays a role in the glucagon counterregulatory response? In fact, an extensive literature search turns up no clinical study results that directly support the amylin circuit-breaker model, presumably because there have been no studies designed to test whether amylin replacement can restore glucagon counterregulation. As for anecdotal evidence among patients using pramlintide, we believe that the absence of supporting data can be attributed to inappropriate dosing of pramlintide, as will be discussed in Part 2.
After a decade on the market, pramlintide has not been considered for restoring glucagon counterregulation. In the early 1990s, preclinical studies suggested that “an amylin agonist may have utility in protecting diabetic individuals from hypoglycemia. However, the spectrum of actions present in rodents was different from those in humans, and this indication was not pursued.” [5] Prior to the development of the amylin circuit-breaker model, it was not plausible to propose that a hormone which suppresses glucagon secretion would be the key player in stimulating glucagon counterregulation.
* * * * * * *
Nevertheless, the question remains: how could pramlintide be used to treat T1D since 2005 and not show any anecdotal evidence of restoring glucose counterregulation? We believe the answer to that question lies in the dosing of pramlintide. As described in Part 2, mealtime doses of pramlintide do NOT provide the tonic inhibition required for the amylin circuit-breaker model to work properly.
Endnotes:
[2] Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients; Journal of Clinical Endocrinology & Metabolism 92:873-9 2007.
[3] Restoration of glucose counterregulation by islet transplantation in long-standing Type 1 Diabetes; Diabetes 64:1713-8 2015.
[4] Phase 3 trial of transplantation of human islets in Type 1 Diabetes complicated by severe hypoglycemia; Diabetes Care 39:1230-40 2016.
[5] Amylin and the integrated control of nutrient influx; Advances in Pharmacology 52:67-77 2005.