Mealtime Dosing of Pramlintide
Is Dysfunctional
The pramlintide dosing instructions for patients with T1D are shown in Exhibit 9.
Patients are advised to inject pramlintide before each meal at the maximum tolerated dose as determined by up-titrating dosing until nausea is experienced, then backing off to the next lower dose.
The pharmacokinetics of pramlintide are comparable to rapid acting insulin. The SYMLIN package insert states that, in healthy subjects, the half-life of SYMLIN is about 48 minutes (Exhibit 10). [1]
Insulin glulisine (APIDRA) has an apparent half-life of 42 minutes, and Exhibit 11 compares the pharmacokinetics of SYMLIN to HUMALOG (fast acting insulin) and HUMULIN (regular insulin). [2]
Because of pramlintide’s relatively rapid pharmacokinetics, about 90% of each pramlintide injection is removed from circulation within 90 minutes post-injection. As shown in Exhibit 12, pramlintide’s suppression of glucagon secretion at the physiologically relevant 30 µg dose has disappeared after about two hours. [3]
How well does pramlintide dosing mimic the healthy diurnal profile of amylin’s plasma concentration? In Exhibit 13, the plot of pramlintide pharmacokinetics shown in Exhibit 10 is overlaid on the healthy plasma profile of amylin shown in Exhibit 4, assuming three mealtime doses daily.
Immediately following injections, pramlintide’s plasma concentration spikes into pharmacological levels, which explains why nausea is the dose limiting adverse event, given that amylin receptors are in the “vomit center” of the brain. Within a couple of hours after injections, pramlintide’s plasma concentration falls to undetectable levels, which eliminates the basal component of amylin’s daily profile, and disables the tonic inhibition of glucagon secretion.
At the highest recommended dose of 60 µg, the total daily pramlintide exposure is only about two-thirds the amylin exposure in healthy, non-diabetic individuals, based on measuring the areas under the diurnal profiles. At 30 µg injections TID pramlintide delivers less than one-third of the normal daily amylin coverage (Exhibit 14).
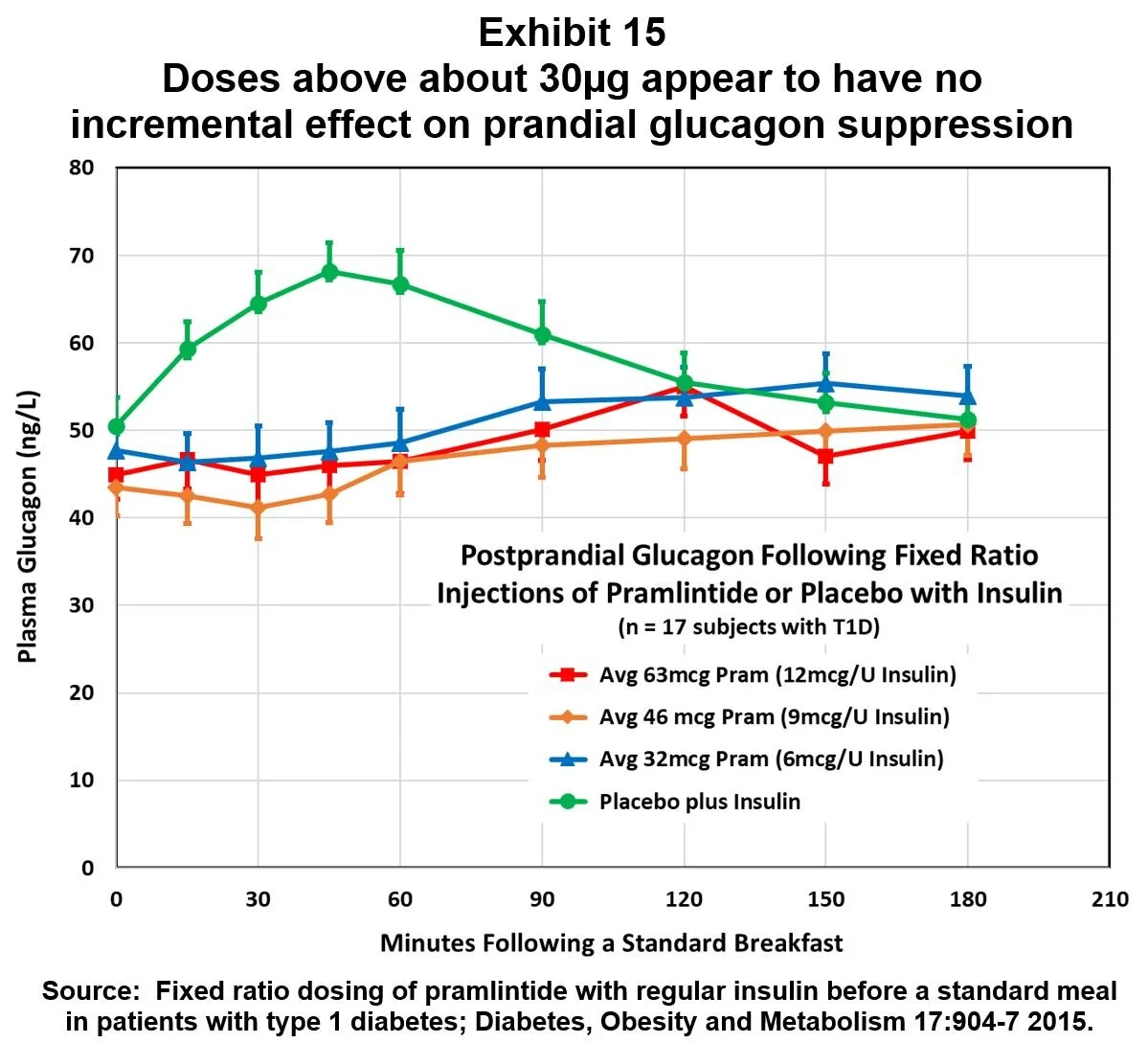
In light of the mealtime overdosing suggested by this analysis, it’s interesting to consider the results of a 2015 study of fixed ratio dosing of insulin and amylin. [4] Three fixed ratios of pramlintide to regular insulin were tested in 17 T1D subjects. Results are shown in Exhibit 15, and they suggest that, on average, the ~30 µg pramlintide dose is at or above the top of the dose/response curve for mealtime glucagon suppression. Consequently, if patients follow the FDA-approved guideline for titrating doses upward until nausea sets in, then many patients are over-dosing pramlintide for prandial glucagon suppression.
In summary, a comparison of plasma levels for pramlintide injections with the diurnal profile of healthy endogenous amylin concentrations indicates that the FDA-approved dosing regimen does NOT mimic the natural hormone. Instead it causes:
Over-dosing at mealtimes, which does not improve glucagon suppression and results in intolerable nausea, and
Under-dosing between meals and especially overnight, which would deactivate the tonic inhibition of glucagon necessary for the amylin switch-off mechanism to activate the glucagon counterregulatory response.
No wonder most T1D patients have found pramlintide to be intolerable and ineffective!
Endnotes:
[1] Pharmacokinetics and Pharmacodynamics of AC137 (25,28,29 Tripro-Amylin, Human) After Intravenous Bolus and Infusion Doses in Patients with Insulin Dependent Diabetes; J Clin Pharmacol 36:13-24 1996.
[2] APIDRA Highlights of Prescribing Information; http://products.sanofi.us/Apidra/Apidra.html.
[3] The human amylin analog, pramlintide, corrects postprandial hyperglucagonemia in patients with type 1 diabetes; Metabolism 51:636-41 2002.
[4] Fixed ratio dosing of pramlintide with regular insulin before a standard meal in patients with type 1 diabetes; Diabetes, Obesity and Metabolism 17:904-7 2015.