Physiology Points to Differences
in Hormone Diurnal Profiles
How could the circulating concentrations of two hormones found in the same secretory granules display different diurnal profiles? We believe the explanation lies in two mechanisms: plasma clearance rates, and beta-cell secretion rates.
Different clearance rates
For diurnally pulsatile hormones, differences in plasma clearance rates would cause variations in circulating molar ratios. Following mealtime pulses, the hormone with a slower clearance rate would retain relatively higher plasma concentrations. In the present case, clearance mechanisms are quite different for the two beta-cell hormones.
Insulin is cleared by the liver, and studies have calculated an average hepatic extraction rate of about 50% of insulin appearing in the portal circulation. [1] It has also been shown that increasing liver exposure to insulin results in decreasing insulin extraction in the liver, which would result in amplifying post-hepatic insulin concentrations (boluses) at higher rates of beta-cell secretion (Exhibit 1). [2]
Amylin, in contrast, is cleared by the kidneys, and mathematical modeling has shown that amylin’s clearance rate is three- to four-fold slower than that of insulin. [3] Amylin’s longer half-life results in a slower rate of decline after mealtime bolus secretions, which would predict relatively higher basal, postabsorptive levels between meals.
These differences in clearance mechanisms and rates between the two beta-cell hormones predict that they should have differing profiles with respect to absorptive vs. postabsorptive states. As stated in a 1998 study of amylin distribution and kinetics: “The lower clearance rate of amylin, which is close to that of C-peptide, as well as the higher mean residence time compared to insulin, indicate that the commonly used insulin-to-amylin ratio is not applicable under non-steady-state conditions.” [4]
Different secretion rates
Beta-cell secretion rates of insulin and amylin may disconnect under certain circumstances. The literature is replete with contradictory evidence about whether the secreted ratio of amylin/insulin can vary; for example:
“We describe two conditions where the release of (amylin) and insulin are dissociated making it unlikely that (amylin) is always co-released from beta-cell granules that contain both peptides and participate only in regulated secretion.” [5]
“Significant differences in the insulin-(amylin) ratios between experimental groups is consistent with the hypothesis that production of (amylin) and insulin are regulated differently in the beta-cell.” [6]
“These results are consistent with the hypothesis that the regulation of (amylin) secretion from beta-cells of isolated rat pancreatic islets is essentially regulated by the same mechanisms as insulin.” [7]
“In summary, it appears that, acutely, the secreted ratio of amylin:insulin is comparatively invariant, but long-standing hyperglycemia may favor induction of amylin synthesis and secretion over that of insulin.” [8]
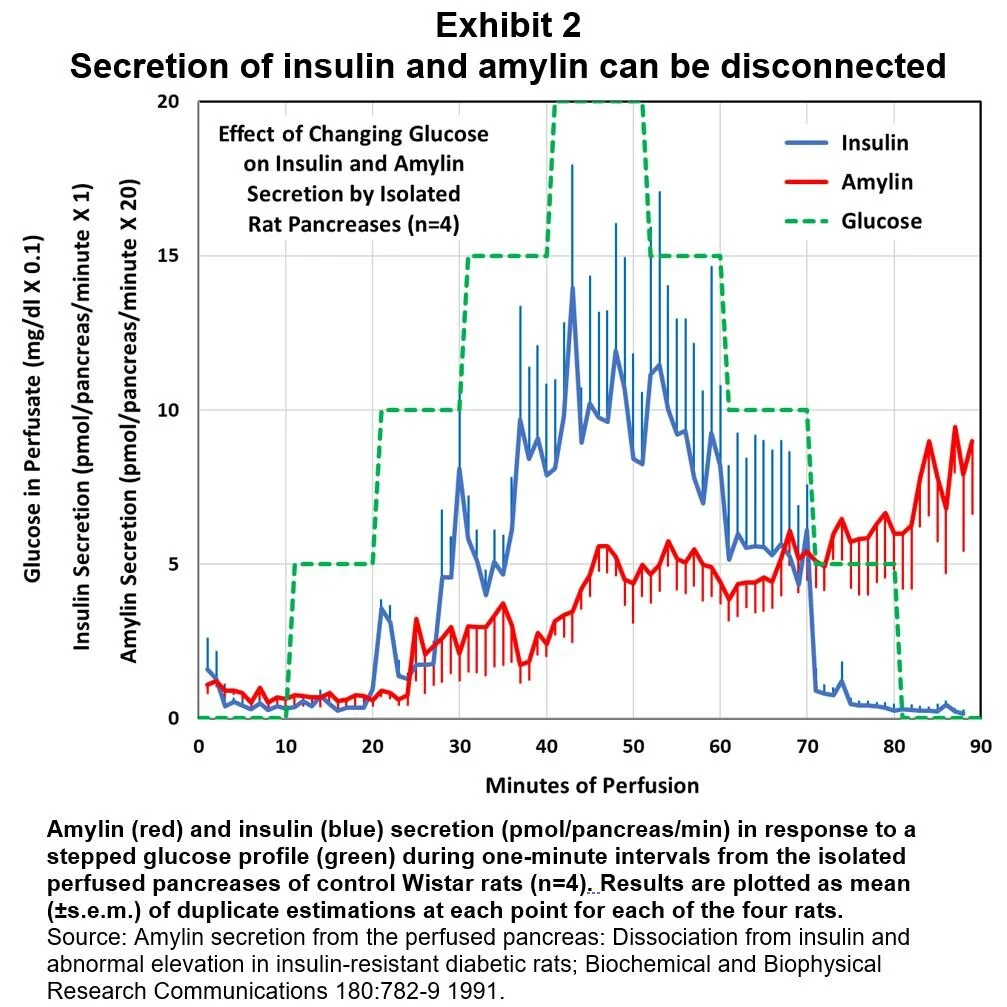
One study of perfused rat pancreases looked at the time course of insulin and amylin secretion when stimulated by stepwise rising and falling levels of perfusate glucose. [9] For normal, non-diabetic rats, insulin secretion closely tracked glucose concentration, while amylin displayed a slowly rising secretion rate (Exhibit 2).
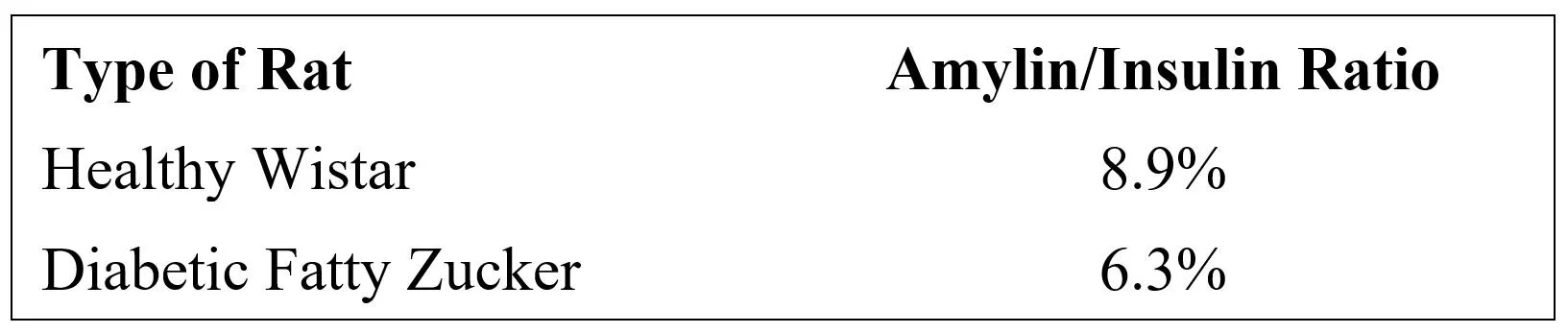
These different rates of response to rising blood glucose would act to vary the prandial peaks of insulin relative to those of amylin as a function of meal size. This study also demonstrated that amylin/insulin molar secretion ratios were different in diabetic rats compared to healthy, control rats:
“These results are the first demonstration of the existence of a mechanism within the pancreas that enables differential secretion of amylin and insulin. In both normal and diabetic rat pancreases, amylin secretion continued to rise after glucose levels in the perfusate fell, whereas in both cases, insulin levels either fell towards basal (normal) or continued to decline (diabetic).” [9]
While these results were in isolated rat pancreases, they are evidence that – under certain circumstances – amylin and insulin secretion rates may become disconnected. And, they suggest that diurnal insulin exposure should be more concentrated in prandial spikes, whereas diurnal amylin exposure should have a larger basal component.
* * * * * * *
In summary, because diurnal beta-cell secretions are pulsatile, differences in clearance rates predict differing profiles of insulin and amylin circulating concentrations. Amylin’s much slower plasma clearance rate predicts relatively higher postabsorptive plasma concentrations. The variable rate of insulin clearance in the liver would amplify prandial insulin peaks in circulation at higher rates of glucose influx without equal amplification of amylin’s profile. Likewise, observed secretion differences predict that more of insulin’s daily exposure would occur during prandial plasma peaks (“boluses”), while amylin’s predicted exposure would be more spread out over the day, thus resulting in relatively higher basal levels of plasma amylin compared to plasma insulin.
What do the data show with respect to diurnal profiles of insulins and amylin?
Endnotes;
[1] A model for the estimation of hepatic insulin extraction after a meal; Trans Biomed Eng 63:1925-32 2016.
[2] Hepatic removal of insulin in normal man: dose response to endogenous insulin secretion; Journal of Clinical Endocrinology and Metabolism 56:1294-1300 1983.
[3] Integrated mathematical model to assess beta-cell activity during the oral glucose test; Am J Physiol 270:E522-31 1996.
[4] Distribution and kinetics of amylin in humans; Am J Physiol 274:E903-8 1998.
[5] Evidence for selective release of rodent islet amyloid polypeptide through the constitutive secretory pathway; Diabetologia 36:570-3 1993
[6] Islet amyloid polypeptide and insulin secretion from isolated perfused pancreas of fed, fasted, glucose-treated, and dexamethasone-treated rats; Diabetes 40:1701-6 1991.
[7] Cosecretion of islet amyloid polypeptide and insulin from isolated rat pancreatic islets following stimulation or inhibition of the beta-cell function; Regulatory Peptides 45:363-70 1993.
[8] Amylin: Physiology and Pharmacology; Advances in Pharmacology Volume 52 2005.
[9] Amylin secretion from the perfused pancreas: Dissociation from insulin and abnormal elevation in insulin-resistant diabetic rats; Biochemical and Biophysical Research Communications 180:782-9 1991.